Use of Ozone to Improve the Safety of Fresh Fruits and Vegetables
In recent years, increasing attention has been focused on the safety of fruits and vegetables, and in particular on the intervention methods to reduce and eliminate human pathogens from fresh produce.
Traditional technology utilizes water with or without a sanitizing agent to wash fresh fruits and vegetables. Chlorine is the most widely used sanitizing agent available for fresh produce, but it has a limited effect in killing bacteria on fruit and vegetable surfaces. The most that can be expected at permitted concentrations is a 1- to 2-log population reduction (Sapers, 1998). Furthermore, the environmental and health communities have expressed concerns about the residual by-products of chlorine.
An alternative treatment is being sought to improve food safety. Research and commercial applications have verified that ozone can replace traditional sanitizing agents and provide other benefits (Bott, 1991; Cena, 1998; Graham, 1997). Many research and industrial trials are underway to validate the use of ozone in the produce industry. Several meetings on this topic have been sponsored by the Electric Power Research Institute (EPRI), including a “Conference on Ozone for Processing Fresh-Cut Fruit and Vegetables” in April 1998 and an “Ozone Workshop” in May 1998. The produce industry is very interested in this technology. However, many questions still have not been resolved, since experience in commercial application in the United States is lacking (Graham, 1997).
Seeking an Alternative to Traditional Sanitizers
In the past two decades, the consumption of fresh fruits and vegetables in the U.S. has dramatically increased. In the meantime, the incidence of foodborne illness due to food pathogens, chemicals, and wastewater has greatly increased. This has been drawing significant public and government attention.
The number of produce-associated foodborne disease outbreaks and the number of cases of illness due to food pathogens have significantly increased in recent years (Tauxe et al., 1997). Moreover, losses in the fresh produce industry that are attributable to microbial spoilage between the time of harvest and consumption are estimated to be as high as 30% (Beuchat, 1991).
Chlorine is commonly used in the fresh fruit and vegetable industry to improve microbiological quality and control pathogens. However, many research studies have indicated that it is limited in its ability to kill bacteria on fruit and vegetable surfaces (Bott, 1991; Cena, 1998; Graham, 1997; Rice et al., 1982; Sapers, 1998). Environmental and health organizations have expressed concerns with traditional sanitizing agents with respect to the formation of by-products, such as trihalo-methanes (THMs) and other chemical residues formed in the wastewater returned to the environment (Anonymous, 1998; Cena, 1998; EPRI, 1997; Graham, 1997). The produce industry is concerned about the possibility of future regulatory constraints on the use of chlorine as a sanitation agent.
--- PAGE BREAK ---
Large amounts of pesticides have been used annually to control insects on fruits and vegetables (Ong et al., 1995). Current technologies cannot totally destroy the chemical residues on the surface of fruits and vegetables. These chemical residues may react with pesticides to form chemical by-products. These residues ultimately will be consumed by customers and may directly and indirectly affect public health. An accumulation of toxic chemicals in the environment has increased the national focus on the safe use of disinfectants, sanitizers, bleaching agents, and other chemicals in the food processing industry.
The produce industry is one of the largest and most important contributors to the world economy. It also generates billions of gallons of wastewater annually, with very high concentrations of biochemical oxygen demand (BOD) and chemical residues each year in the U.S. These wastewaters have been linked to many serious problems such as cancer, fish death, water pollution, psychological and physiological diseases, and ecosystem damage. Moreover, the produce industry is paying heavy charges and surcharges for discharging wastewater into public water and wastewater treatment systems (Carawan, 1999).
In response to the public concerns about food safety, the President of the United States and Congress issued a new federal initiative in 1997—the President’s Food Safety Initiative—to improve the nation’s food safety system and our environment. One of the approaches to improve food safety is to identify an alternative sanitizer to replace traditional sanitizing agents which can also be used to treat or recycle food processing wastewater.
Research and commercial applications have indicated that ozone can replace chlorine with more benefits. In 1997, ozone was self-affirmed as Generally Recognized As Safe (GRAS) as a disinfectant for foods by an independent panel of experts sponsored by EPRI (Graham, 1997). This self-affirmation was timely for the produce industry in light of the President’s Fruit and Vegetable Safety Initiative. The produce industry is very interested in the use of ozone and would like to know how, when, and where to apply it.
Why Ozone?
The potential utility of ozone in the produce industry depends on the fact that as an oxidizing agent, it is 1.5 times stronger than chlorine and is effective over a much wider spectrum of microorganisms than chlorine and other disinfectants. Ozone kills bacteria such as Escherichia coli, Listeria, and other food pathogens much faster than traditionally used disinfectants, such as chlorine, and is free of chemical residues (Langlais et al., 1991; Sapers, 1998).
Ozone is a high-energy molecule. Its half life in water at room temperature is only 20 min, and it decomposes into simple oxygen with no safety concerns about consumption of residual ozone in the treated food product (Graham, 1997). It can also be used for recycling water (Anonymous, 1998; Perkins, 1997).
--- PAGE BREAK ---
Fresh fruits and vegetables are washed first by ozonated water, and the wash water can be recaptured and treated by a combination of ozonation and filtration. The treated wash water is free of bacteria, color, and suspended solids and can be recycled to reduce water usage. Unlike conventional chlorine-based washing systems, wastewater discharged by an ozonation process is free of chemical residues, a growing concern related to the environment and groundwater pollution (Anonymous, 1998). Ozone can also destroy pesticides and chemical residues, such as chlorinated by-products (Langlais et al., 1991).
Gaseous ozone is a strong sanitation and fumigation agent and can be used to sanitize foods in the storage room and during shipping to prevent bacteria, mold, and yeast on the food surface and to control insects. It can eliminate undesirable flavor produced by bacteria and chemically remove ethylene gas to slow down the ripening process, thus allowing extended distribution (Rice et al., 1982).
For decades, it has been known that ozone is an effective disinfectant and sanitizer for the treatment of food products. It is commonly used in Europe for treatment of public water systems and food processing. It is being used in the U.S. for bottled water and has the potential for use in many food processing applications. Numerous documents and studies confirm the benefits of ozone applications in the food industry (Graham, 1997; Rice et al., 1982). Thus, ozone can successfully replace traditional sanitizing agents to control food pathogens.
Ozone is triatomic oxygen, a naturally occurring form of oxygen that was first identified in 1840:
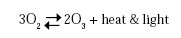
It is partially soluble in water and, like most gases, increases in solubility as the temperature decreases. It is effective in killing microorganisms through oxidation of their cell membranes (Langlais et al., 1991). Ozone has a unique property of autodecomposition and will leave no toxic residues (Neff, 1998). It has an oxidation potential 1.5 times stronger than that of chlorine and has been shown to be effective over a much wider spectrum of microorganisms than chlorine and other disinfectants.
Ozone is generated naturally by ultraviolet irradiation from the sun and from lightning. It can be generated commercially by UV lights (at 185 nm) or corona discharge. If a high concentration of ozone is desired, corona discharge is commonly used. There are two types of feed gas—air, generally at a concentration of 1–3% (w/w), and oxygen, generally at 2–12% (w/w) (Pryor, 1998).
--- PAGE BREAK ---
Applications in the Produce Industry
Many applications in the produce industry appear suitable for the use of ozone:
• Process Water Sterilization. Over the past several years, there has been increasing evidence that process water used by the food industry is not as free of pathogens as previously thought. Additionally, there are many situations in which process water is cross-contaminated either before or during the process. In these cases, disinfection and sterilization treatment must be applied to maintain acceptable low levels of microorganisms that may come in direct contact with the food. Moreover, there is a certain level of pesticide and toxic organic compounds in the process water supply due to industrial activities.
Normally, processing water is disinfected and sterilized using chlorine. However, chlorine cannot reduce the level of organic compounds and will produce chlorinated compounds. Ozone has been proven to be an ideal replacement for chlorine for disinfection and sterilization of process water (Geering, 1999; Langlais et al., 1991; Rice, 1999).
According to the Environmental Protection Agency, ozone is the most effective primary disinfectant available for drinking water. In fact, it is more effective than chlorine against microorganisms, including chlorine-resistant Cryptosporidium and Giardia which have invaded the food and water supplies and caused deaths in recent years (EPRI, 1997; Hoff, 1997). The Ct value for 99% inactivation of Cryptosporidium is less than 2 mg min/L for ozone and higher than 30 for chlorine. Ct is defined as the product of the disinfectant concentration and the time required to achieve a given level of a microorganism exposed under defined conditions (Langlais et al., 1991).
Ozone can also destroy chlorine byproducts, pesticides, and toxic organic compounds in the process water without any toxic residues (Langlais et al., 1991). Practical applications of ozone to process water range from 0.5 to 5 ppm (depending on the water source), with less than 5 min contact time.
Ozone is also used to remove iron, manganese, and sulfur and to control taste and odor of fresh water. This application will continuously maintain high-quality water free of microorganisms and toxic chemicals for the produce industry.
• Fruit and Vegetable Washing. One way to maintain or even improve the safety of fresh produce is to wash vegetables and fruits using ozonated water (Hampson and Fiori, 1997). Two types of washing systems, spray and flume, can be used to reduce microbial counts on the surface of produce. Kim et al. (1999) used ozonated water to wash shredded lettuce. They injected 1.3 mM of ozone at a flow rate of 0.5 L/min into a water/lettuce mixture (1:20, w/w) with high-speed stirring or before stomaching for 3 min and obtained about 2 log cfu/g reduction in total plate counts. Kondo et al. (1989) obtained >90% reduction of total bacterial counts for Chinese cabbage by this method. Ozone is particularly effective against E. coli, the food pathogen of most concern to the produce industry.
• Fruit and Vegetable Storage. Ozone can be employed in cold storage of produce to guard against mold and bacteria at a very low concentration. It can not only destroy mold and bacteria in the air and on the surface of produce but also deodorize (Rice et al., 1982).
Many early studies used gaseous ozone to prevent microbial activity on food surfaces and extend the shelf life of fruits and vegetables. Since 1933, numerous experiments have been done on a wide variety of fruits and vegetables, including apples, potatoes, tomatoes, strawberry, broccoli, pears, cranberries, oranges, peaches, grapes, corn, and soybeans (Perkins, 1997; EPRI, 1997).
--- PAGE BREAK ---
Barth et al. (1995) assessed ozone exposure on storage of thornless blackberries. Blackberries were harvested and stored at 2ºC in air with 0.3 ppm ozone. Fungal development was suppressed, while 20% of control fruits showed decay. Ozone treatment did not cause observable injury defects, and surface color was retained for 12 days.
Gaseous ozone treatment could be a good choice for extending the shelf life of strawberries because they are easily damaged by water. Ewell (1940) indicated that the shelf life of strawberries, raspberries, currents, and grapes could be doubled if 2–3 ppm of gaseous ozone is applied continuously for a few hours per day. However, Norton et al. (1968) concluded that 0.6 ppm of ozone at 60ºC caused damage on Early Black and Howes varieties of cranberries. Further studies are needed using low temperature to confirm whether ozone can control fungus with a less detrimental effect.
Kuprianoff (1953) found that the shelf life of apples could be increased by several weeks by applying 2–3 cm3 of ozone/m3 of air a few hours a day. However, ozone concentrations of 10 cm3/m3 resulted in apple damage.
Baranovskaya et al. (1979) pointed out that the shelf life of potatoes could be extended to as long as 6 mo at 6–14ºC and 93–97% relative humidity with 3 ppm of ozone, without affecting the potato quality.
One of the important effects of ozone in cold storage is to slow down the fruit and vegetable ripening process. During ripening, many fruits, such as bananas and apples, release ethylene gas, which speeds up the ripening process. Ozone is very effective in removing ethylene through chemical reaction to extend the storage life of many fruits and vegetables (Rice et al., 1982):
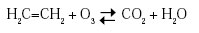
• Process Water Recycling. It is estimated that more than 50 billion gallons of fresh water are used by the produce industry annually (Carawan, 1999). A need exists to decrease the quantity of water used, because of dramatically rising water and wastewater treatment costs, difficulties in obtaining large water volumes, highly variable water supplies, and problems of wastewater treatment and disposal. Ozone is a perfect candidate for treatment of water for recycling, since it is a powerful oxidizing agent that has been used to disinfect, to remove color, odor, and turbidity, and to reduce the organic loads of wastewater (Geering, 1999; Langlais et al., 1991; Rice, 1999).
Williams et al. (1995) reported that a 3-log reduction of bacteria was achieved when the wash water from carrots was treated by ozone. Piper (1998) showed that tomato washing using ozonated water dramatically improved the bacterial quality. The wash water was recycled at a very high quality (light transmission >95%, scale formation <0.01 in/year, and corrosion rates less than 5 m/year for mild steel).
There are many studies and applications using ozone to recycle processing water in other industries, particularly the poultry industry. Several commercial technologies are currently available, such as the Praxair and Zentox Water-Treatment Alliance. Further research is needed on wastewater from the produce industry.
The equipment used in ozone applications in the produce industry is relatively simple. Complete ozone systems with water recycling include generators (size depending on the applications), contact tanks, de-gas system, ozone-destruct units, filters, ozone monitors, and exhaust system. The system can be designed to fit a small area and can be very easily installed without any major modifications of the processing lines.
--- PAGE BREAK ---
Ozone Safety
Ozone is formed naturally in the upper atmosphere from oxygen by UV light and by atmospheric electrical discharges such as lightning or the aurora borealis. It is also found in lower levels of the atmosphere, primarily as a result of photochemical oxidation of hydrocarbons from automobile and industrial emissions. It is also coincidentally produced by photocopiers, electrical transformers, and other electrical devices. Humans are exposed to low levels of ozone on a daily basis (Pryor, 1998).
Like all oxidizing gases, it is potentially harmful if human exposure occurs at high concentrations for a sufficient duration. Threshold limits have been established by the Occupational Safety and Health Administration. The current Threshold Limit Value–Long Term Exposure Limit (TLV-LTEL) for ozone exposure in the workplace environment is 0.1 ppm for a normal 8-hr day/40-hr work week, as recommended by the American Conference of Governmental Industrial Hygienists (ACGIH) and approved by OSHA. The current Threshold Limit Value–Short Term Exposure Limit (TLV-STEL) is 0.3 ppm for 15 min. This is the level to which healthy individuals can be exposed for a short period of time (15 min) without suffering from physical irritation or other acute effects, provided that the TLV-LTEL is not exceeded.
Ozone has the lowest TLV-LTEL value, compared to other commonly used gases such as CO2, N2, and O2. It is safer to use than other gases (Pryor, 1998) because it:
• Has faster reaction kinetics because of its very high oxidation potential. Either less of the chemical or reduced contact times are needed to complete the desired oxidation reactions, compared to weaker oxidizing agents.
• Is generated onsite, at relatively low concentrations and pressures (<15 psig). It is immediately consumed in the treatment process and cannot be stored as a compressed gas. Unlike other gases, an uncontrolled, widespread, and sudden release of large quantities of ozone is not possible.
• Has a relatively short half-life, generally measured in minutes in the aqueous phase to hours in the gas phase. Any accidental release of ozone will not persist in the environment for a long period of time, compared to a release of a more stable toxic gas.
• Decomposes into simple diatomic oxygen upon breakdown. It will not form environmentally harmful or persistent compounds upon reaction with common hydrocarbons, nor will it result in the formation of chlorinated hydrocarbons such as THMs.
• Has a characteristically strong odor. It can be sensed at concentrations as low as 0.01 ppm, or one-tenth of the allowable TLV-LTEL. It is easily detected by an individual at very low concentrations before a harmful situation is reached. Ozone is considered to be freely dispersed in the atmosphere according to EPA models. It will not sink to low levels and concentrate near the ground, where the human exposure potential is greatest.
--- PAGE BREAK ---
• Exerts only temporary, acute symptoms upon human exposure, Except in very rare cases of extended, severe overexposure to a high concentration (several hours at >2–3 ppm), the physical symptoms of ozone exposure are acute and transitory in nature. These symptoms include watery eyes, tightness in the chest, shortness of breath, and irritated throat. Headaches or lightheadedness are possible. Recommended treatment of excessive exposure includes removal of the exposed personnel from the exposure area and rest, except in severe cases of overexposure where application of oxygen is recommended. Symptoms generally begin to subside within minutes once the exposure is ended, and complete recovery occurs within hours or days, even in the most severe exposure cases.
Ozone is not characterized as a carcinogen or mutagen. It does not accumulate in fatty tissue or cause long-term chronic effects (Pryor, 1998).
From the above discussion, it is clear that ozone can be used safely. However, measures must be taken when working with ozone to prevent unnecessary exposure.Proper personnel protective equipment, exhausting system, destruct unit, and monitors should be used when working with ozone.
Implementing Ozone Technology
To safely adopt ozone technology, a company should do the following before making any major capital investment:
• Understand the process flow, to know exactly where ozone will fit in and why.
• Conduct pilot trials before starting commercial application, because every ozone application is unique. Ozone efficiency will be affected by many factors, such as water quality, temperature, pH, and composition of products. Pilot testing will help the engineer to determine the size of the generator and the costs of the system.
• Know the water and wastewater parameters. If processors want to recycle process water (it is always a good practice to use ozonated water to wash produce, if it is to be recycled), knowing the plant water and wastewater parameters will be useful in designing the system.
• Work with an ozone company experienced in the produce industry, since ozone applications there are significantly different from those in other industries, such as water treatment and the laundry industry. The partner selected to work with should be able to identify opportunities; help conduct ozone testing; provide applications technology expertise on produce; conduct cost analysis; provide reliable equipment; recommend ozone level and contact time; have expertise in ozone production and injection; have the ability to design and install a commercial system; and understand the regulatory and safety issues to assure compliance and environmental and public health.
